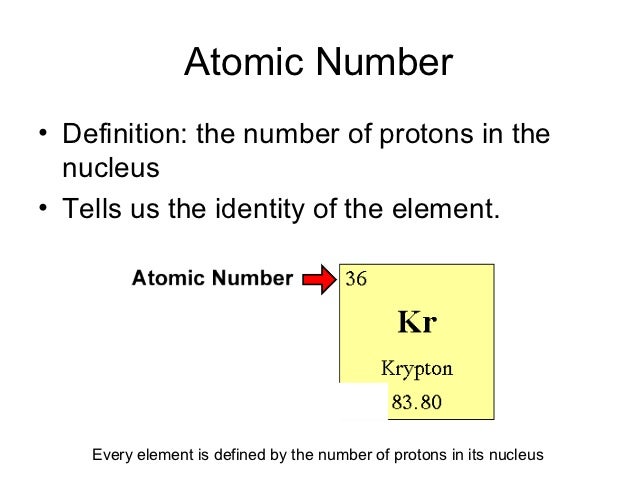
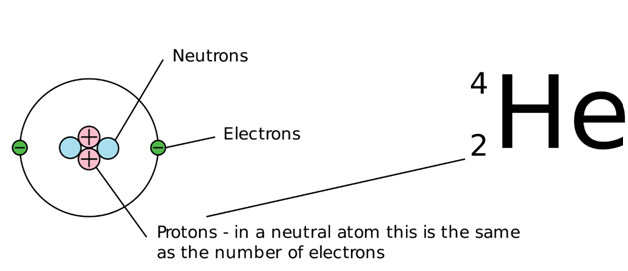
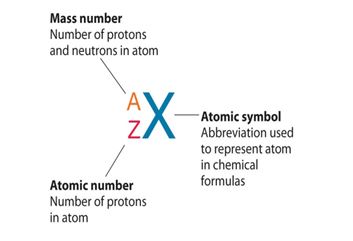
Definition Of Atomic Number
/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)
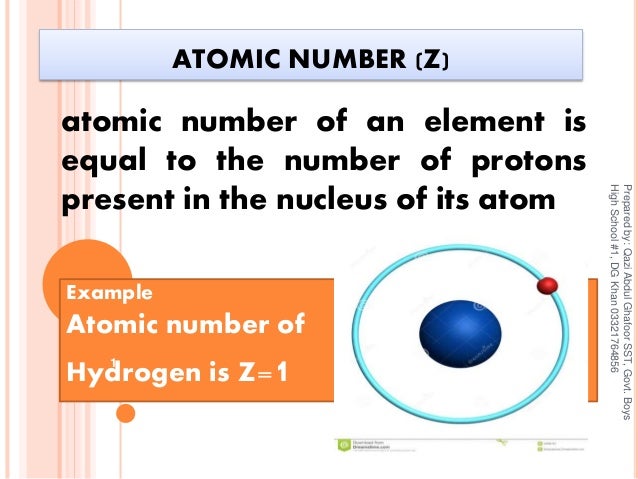
Atomic number definition is an experimentally determined number characteristic of a chemical element that represents the number of protons in the nucleus which in a neutral atom equals the number of electrons outside the nucleus and that determines the place of the element in the periodic table.
Definition of atomic number. In electrically neutral atoms this number is also equal to the number of electrons orbiting about the atom s nucleus. The number of protons in the nucleus of an atom. The number of protons or electrons normally found in an atom of a given chemical element. The higher the atomic number the heavier the atom is.
In a neutral atom the number of protons and electrons is the same.
Source : pinterest.com






/atomic-structure-artwork-549603139-57fe40e75f9b586c3537ebf4.jpg)